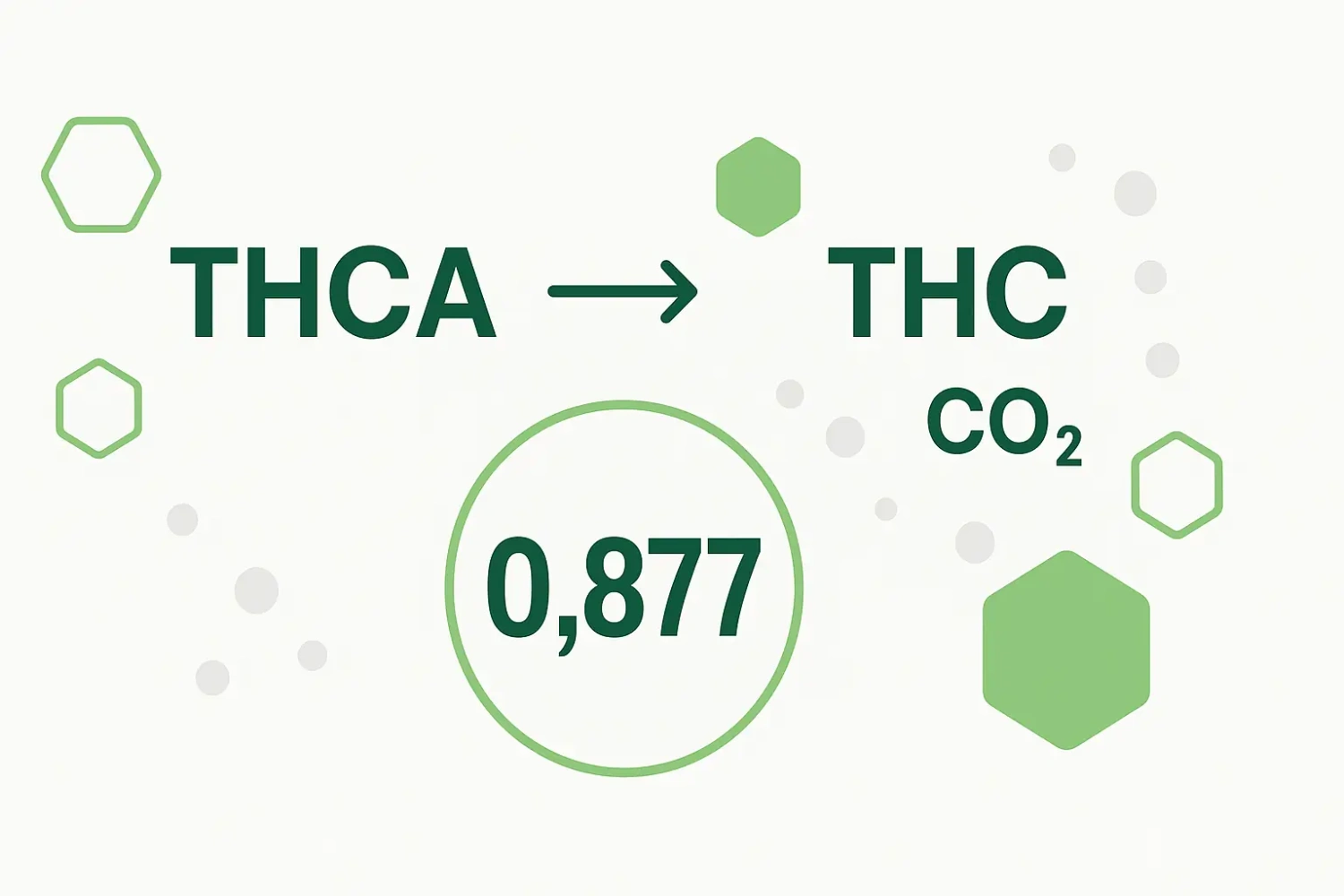
Calculation of total available THC and the number 0.877
The number 0.877 is not random at all, but where does it come from? Simply put, it originates from the structure of cannabinoid molecules.
As you may know, the plant versions of the main cannabinoids, sometimes called cannabinoid acids, must be “decarboxylated” to gain their active effects. This decarboxylation is called a decomposition reaction—one molecule becomes two. In our case, one of these molecules is always CO2 (carbon dioxide is the source of the decarboxylate). The second molecule is the “active” or “neutral” cannabinoid itself.
The number 0.877 is actually constant and is based on the ratio of the weights of the cannabinoid molecules. Most of the major cannabinoids (THC, CBD, CBG, CBC, but not CBN) have the same molecular formula: C21H30O2, for 21 carbon atoms, 30 hydrogen atoms, and 2 oxygen atoms. Equivalent cannabinoid acids (THCA, CBDA, CBGA, and CBCA) are “neutral” cannabinoids that “carry” a CO2 molecule and change their molecular formula to C22H30O4 with the addition of one carbon and two oxygen atoms.
Each element in a molecule has a measurable weight, and the most common weight of an element is usually the largest number in the element’s field in the periodic table. Carbon has an atomic weight of approximately 12.011, hydrogen approximately 1.008, and oxygen almost exactly 16.
We can calculate how much each molecule of THC weighs in the following way:
Molecular weight of THC = 21 x carbon (12.011) + 30 x hydrogen atom (1.008) + 2 x oxygen (16.000)
Molecular weight of THC = 314.47
We can calculate the same for THCA:
Molecular weight of THCA = 22 x carbon (12.011) + 30 x hydrogen atom (1.008) + 4 x oxygen (16.000)
Molecular weight of THCA = 358.48
The molecule released during “decarboxylation,” CO2, has a molecular weight of about 44.01. If we add THC 314.47 and CO2 44.01, we get a molecular weight of THCA 358.48. The universe makes sense! So far, so good. If we take it one step further, we realize that THCA is not actually all of the THC. It is only 314.47 / 358.48 = 0.8772 or 87.72%. And that’s our number 0.877! The remaining 12.28% is CO2, which escapes as a gas during decarboxylation—this process is illustrated, for example, by bubbling dab on a hot nail.
Now, the goal of calculating available THC is to find the maximum amount of “active” THC under absolutely ideal conditions that can be obtained from the sample. If THCA is only 87.72% THC, we take this into account in our calculation of available THC. Multiply the amount of THCA by 0.877 before adding it to the amount of already activated THC. In other words, a gram of 100% pure THCA contains 0.877 grams of THC and 0.123 grams of CO2.
The same “activation multiplier” can be used to calculate the available CBD, CBG, or any other cannabinoid with the molecular formula C21H30O2. You may know that the US Herbal Pharmacopoeia uses a multiplier of 0.878 for CBGA when calculating available cannabinoid content. This is because the molecular structure of CBGA contains two additional hydrogen atoms for the formula C22H32O4. When CBGA decarboxylates to CBG, the two hydrogen atoms are retained, and CBG therefore has two more hydrogen atoms in its structure than THC or CBD. The same math with the molecular weights of CBGA and CBG (360.75 and 316.74) gives a conversion factor of 0.8780, which is slightly different from 0.877 for the conversion of THCA to THC.
For the group of cannabinoids ending in -varin, of which THCV is the best known (but which also includes CBDV, CBGV, CBCV and their respective acids CBDVA, CBGVA, etc.), we need a different activation number because the molecular weights are not the same: cannabivarins are missing two carbons compared to their common cannabinoid cousins. etc.), we need a different activation number because the molecular weights are not the same: cannabivarins are missing two carbons and four hydrogens compared to their common cannabinoid cousins, giving us molecular formulas of C19H26O2 (weight 286.42) and C20H26O4 (weight 330.43) for their acids. Our “activation multiplier” for the -varin group is 0.8668 instead of 0.8772. It’s close, but not the same!
Hopefully, this text has answered some of your questions about this popular herb, or perhaps sparked your interest in learning more about chemistry.
Summary: The seemingly random number 0.877 is the ratio of molecular weights, specifically the weight of THC divided by the weight of THCA. If you multiply the amount of THCA by 0.877 and add the amount of already “active” THC, you will find the maximum amount of THC remaining after complete decarboxylation. THCA is about 87.7% THC and 12.3% CO2 by weight.
Photo: iStock
“All information provided on this website, as well as the information provided through this website, is for educational purposes only. None of the information contained herein is intended as a substitute for medical diagnosis and such information is not to be considered medical advice or recommended treatment. This website does not promote, endorse or advocate the legal or illegal use of narcotic drugs or psychotropic substances or the commission of any other illegal activity. Please see our Disclaimer for further information.“


